Calculator Plugins History of Changes
18th May 2020, Chemical Terms, 20.13
New features and improvements
18th May 2020, Calculator Plugins, 20.13
Bugfixes
-
Wiggly bonds are put in the generated major tautomer forms of the some molecules with defined E or Z bonds
New features and improvements
-
Show tautomer region tautomer generation option in the Tautomerization Plugin in MarvinSketch
27th April 2020, Calculator Plugins, 20.12
Bugfixes
10th March 2020, Calculator Plugins, 20.8
New features and improvements
- Option for setting the correction library path for the pKa and logP training files in the logD node of Pipeline Pilot
18th December 2019, Calculator Plugins, 19.27
Bugfixes
-
Tautomerization/tautomer generation fails for a molecule with valence error
-
Differences in logD calculation results with and without the Consider tautomerization option for some molecules with only one dominant form
New features and improvements
-
Take tautomerization into account during IEP calculation with the IEP Plugin: IEP is calculated for the major tautomer form of the input molecule
13th November 2019, Calculator Plugins, 19.24
Bugfixes
5th August 2019, Calculator Plugins, 19.19
Bugfixes
- Exceptions and errors in generic tautomer generation for a set of aromatic and heteroaromatic agents
11th July 2019, Calculator Plugins, 19.17
New features and improvements
- New Required HLB calculation in the HLB Predictor and its integration into MarvinSketch and cxcalc
20th June 2019, Calculator Plugins, 19.14
Bugfixes
-
NullPointerException is thrown for some D-substituted compounds during standardisation
-
Valence checking error causes normal canonical tautomer generation to fail during standardisation of some salt compounds
New features and improvements
-
Varying assignment of close pKa values in symmetric molecules with many pKa values
30th May 2019, Calculator Plugins, 19.12.0
Bugfixes
17th May 2019, Calculator Plugins, 19.11.0
New features and improvements
- Improved MolecularFormulas and Elemental Analysis API
3rd May 2019, Calculator Plugins, 19.10.0
Bugfixes
29th March 2019, Calculator Plugins, 19.9.0
Bugfixes
-
Incorrect generic tautomer pairs where generated during tautomer search for certain molecules
New features and improvements
-
Improved pKa and tautomer calculations for certain molecules
25th February 2019, Calculator Plugins, 19.6.0
New features and improvements
- the Protect Charge option of the tautomer generation is automatically adjusted in the case of dynamic pKa calculation
11th February 2019, Calculator Plugins, 19.4.0
Bugfixes
-
Quaternary pyridine effect on phenol is improved for the pKa calculation
-
Improved canonical tautomer generation
-
Incorrect generated normal canonical form is fixed for certain molecules
18th January 2019, Calculator Plugins, 19.2.0
Bugfixes
-
LogD calculation with the Consider tautomerization option does not terminate for casein
-
Compound chemical formulas of molecules without C atoms are not listed alphabetically
New features and improvements
-
Improving the predicted pKa values of certain CH acids
-
Weights of elements in the elements.txt file (used by the Elemental Analysis Plugin) are updated to the IUPAC 2017 values
13th December 2018, Calculator Plugins, 18.30.0
Bugfixes
-
Elemental Analysis Plugin handles repeating unit groups incorrectly during calculation
-
Incorrect standardized forms of some ChemBL compounds and salts
-
Strongest basic pKa value of cytosine needs some correction based on experimental data
-
Fixing some Exception in charge calculations during standardization
-
Inconsistent pKa values of ionised and neutral form of boronic acids
30th October 2018, Calculator Plugins, 18.25.0
Bugfixes
- Microspecies table of the pKa Plugin does not change when pKa is calculated at different temperatures
5th September 2018, Calculator Plugins, 18.22.0
Bugfixes
24th August 2018, Calculator Plugins, 18.21.0
Bugfixes
-
Amine compounds lose stereo flags during tautomerization in standardization processes (in Standardizer)
-
H atom is added to the generated resonant structure of heterocyclic compounds during standardization (in Standardizer)
-
Incorrect standardized (normal canonical tautomer) forms of stereoisomers of sulphur compounds
1st August 2018, Calculator Plugins, 18.18.0
Bugfixes
-
De-aromatization of a large ring with N fails
-
Standardization (tautomerization) results of the same molecule coming from DB sources are different
17th July 2018, Calculator Plugins, 18.17.0
Bugfixes
7th June 2018, Calculator Plugins, 18.13.0
Bugfixes
- Elemental Analysis Plugin calculates properties for molecules with position variation bonds incorrectly
18th May 2018, Calculator Plugins, 18.11.0
Bugfixes
-
Inconsistent pKa values with changing min/max calculation options
-
Inconsistent isoelectric point calculation for iminodiacetic acid
-
Incorrect isoelectric point calculation for omeprazole
9th March 2018, Calculator Plugins, 18.8.0
Bugfixes
-
Wrong generic tautomer forms in some cases
New features and improvements
-
Improving the pKa calculation and the logD values for some bioisosteric compounds
10th January 2018, Calculator Plugins, 18.1.0
New features and improvements
-
Adding Recognize pseudo formula in pseudo labels option to the ElementalAnalyzerPlugin node in KNIME
Bugfixes
-
Topology Analysis outputs incorrect values for some molecules
6th December 2017, Calculator Plugins, 17.29.0
Bugfixes
-
Solubility Predictor calculates different solubility values for the same molecule in neutral and ionized forms
-
Generic tautomer forms of a molecule pair with negative charge are incorrect
-
Exception is thrown during pKa calculation for tricyclic bromine compounds
23rd October 2017, Calculator Plugins, 17.27.0
New features and improvements
- Calculating molecular weight from molecular formula. See the API doc page of this feature.
16th October 2017, Calculator Plugins, 17.26.0
Bugfixes
-
Incorrect tautomerization of some heterocyclic molecules during standardization
18th September 2017, Calculator Plugins, 17.23.0
Bugfixes
28th August 2017, Calculator Plugins, 17.20.0
Bugfixes
-
Different dominant tautomer distribution of the same molecule with a rotated bond
7th August 2017, Calculator Plugins, 17.18.0
Bugfixes
3rd July 2017, Calculator Plugins, 17.14.0
New features and improvements
-
The Isoelectric Point Plugin is improved so that it handles quaternary amine salts as a microspecies carrying +1 charge (instead of neutral)
-
Modifying table text of the exported PDF file in the Solubility Predictor
Bugfixes
-
Major tautomer calculation at pH 7.4 throws exception for specific molecules
-
pKa calculation in cxcalc throws exception for a specific molecule
3rd July 2017, Chemical Terms, 17.14.0
New features and improvements
- maximalprojectionsize and minimalprojectionsize functions are introduces in Chemical Terms
30th May 2017, Calculator Plugins, 17.10.0
Bugfixes
-
Tautomer pair has slightly different generic tautomers
-
Predicted pKa value of sorbic acid is off in the new Chemicalize (requires fine-tuning)
3rd May 2017, Calculator Plugins, 17.7.0
Bugfixes
-
pKa values of the N1 atom of adenosine predicted with the new version of Chemicalize were quite off compared to the experimental results
New features and improvements
-
Only practically justified isoelectric points (when the input molecule has a certain pKa and charge distribution threshold) will be printed on the output of the Isoelectric Point Plugin
10th April 2017, Calculator Plugins, 17.04.10.
Bugfixes
6th March 2017, Calculator Plugins, 17.03.06
New features and improvements
- Re-placing the Resonance Plugin under the Isomers plugin group
27th February 2017, Calculator Plugins, 17.02.27
Bugfixes
-
Incorrect pKa and pH-dependent solubility predicted for the indigo (CAS 482-89-3) molecule
-
Wrong output after filtering generated 3D stereoisomers in the StereoIsomer Plugin
-
Input molecule (with the predicted properties) does not appear correctly in the output window of some protonation-type plugins (e.g. pKa, HBDA)
-
Incorrect Elemental Analysis calculations results (e.g. mass, formula) for larger molecules with S-groups
-
Alignment Plugin throws an exception when there are no input molecules on the canvas
20th February 2017, Calculator Plugins, 17.02.20
Bugfixes
-
Incorrect tautomer distribution predicted for some thioamide compounds in the Tautomer Plugin
-
Incorrect tautomer distribution predicted for some nitroparaffin compounds in the Tautomer Plugin
-
Incorrect normal canonical tautomers calculated for some heterocyclic compounds in the Tautomer Plugin
-
Input molecules don't appear in the result window of the NMR Predictor. The molecules only appeared after hitting the Update button of the viewer.
-
Input molecule appears small and translated in the result window of the Solubility Predictor even after pressing the Update button of the viewer.
13th February 2017, Calculator Plugins, 17.02.13
New features and improvements
- Charged and isotope atoms are recognized in formula during Elemental Analysis calculations (e.g. mass)
23rd January 2017, Chemical Terms, 17.01.23
New features and improvements
-
New Largest Conjugated System calculation functions are available in Chemical Terms
-
New Chemical Terms demo site is available
16th January 2017, Calculator Plugins, 17.01.16
Bugfixes
9th January 2017, Calculator Plugins, 17.01.09
Bugfixes
2nd January 2017, Calculator Plugins, 17.01.02
Bugfixes
26th December 2016, Calculator Plugins, 16.12.26
Bugfixes
-
Incorrect results with stereoisomer generation for some molecule
-
Some modifications in the StereoisomerSettings API
19th December 2016, Calculator Plugins, 16.12.19
Bugfixes
-
Strange generic tautomer forms for some molecules
-
Incorrect tautomer distributions for different tautomer forms of the same molecule
21st November 2016, Chemical Terms, 16.11.21
New features and improvements
- Introducing name export options for the name() function
14th November 2016, Calculator Plugins, 16.11.14
Bugfixes
-
Incorrect pKa values are calculated for MINA and DAM (acetone derivative) compounds
-
OutOfMemoryError was thrown during conformer generation
17th October 2016, Calculator Plugins, 16.10.17
Bugfixes
-
Solubility values for the same molecule represented by different SMILES codes were different
10th October 2016, Chemical Terms, 16.10.10
Bugfixes
- Incorrect dominant tautomer forms are generated for some azide-type molecules using the dominantTautomer() function
3rd October 2016, Chemical Terms, 16.10.3
Bugfixes
- Incorrect dominant tautomer forms are generated for some molecules containing Se using the dominantTautomer() function
5th September 2016, Calculator Plugins, 16.9.5
Bugfixes
-
Incorrect logP atomic increments are calculated by the getAtomlogPIncrement() function for some molecule
New features and improvements
-
Large molecules make the pH-dependent solubility calculation slow down. Calculation was speeded up.
29th August 2016, Chemical Terms, 16.8.29
Bugfixes
- Incorrect dominant tautomer forms and distributions are generated using the dominantTautomer() function
29th August 2016, Calculator Plugins, 16.8.29
Bugfixes
-
Incorrect isotope handling during tautomerization: isotope atoms vanish in result tautomer forms
18th July 2016, Calculator Plugins, 16.7.18
Bugfixes
-
Carbanions appear in the major microspecies form at pH 7.4 with some molecules in the Major Microspecies Plugin
4th July 2016, Calculator Plugins, 16.7.4
Bugfixes
13th June 2016, Calculator Plugins, 16.6.13
Bugfixes
-
Atomic charges don't add up to total charge in the Charge Plugin for some molecule
-
Normal canonical tautomer generation throws Exception for some molecules during standardization
-
Incorrect standardized form of cyanidine due to wrong tautomerization
10th May 2016, Calculator Plugins, 16.5.10
Bugfixes
- Elemental Analysis Plugin calculates basic properties for molecules with double variation bonds incorrectly
3rd May 2016, Calculator Plugins, 16.5.3
New features and improvements
- Some properties and calculation option are removed from the Elemental Analysis Plugin, and the plugin window is re-organized based on some user feedback
April 25th 2016, Calculator Plugins, 16.4.25
Bugfixes
-
Precision of calculations does not work as expected in the Analysis Box in MarvinSketch
April 18th 2016, Calculator Plugins, 16.4.18
New features and improvements
-
Groups written in pseudo labels are recognized in Elemental Analysis calculations
May 16th 2016, Calculator Plugins, 16.5.16
Bugfixes
- Incorrect tautomerization of some pyridine derivatives
February 22nd 2016, Calculator Plugins, 16.2.22
Bugfixes
January 18th 2016, Calculator Plugins, 16.1.18
Bugfixes
- Incorrectly calculated pKa values for the molecule with CAS number 1224844-38-5.
January 11th 2016, Calculator Plugins, 16.1.11
New features and improvements
- New feature in the Tautomerize Plugin: canonical tautomerization of organic molecules containing metal atoms. See an example of tautomer pairs below:
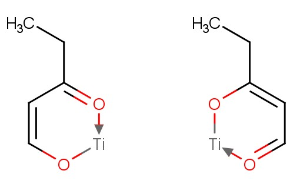
January 11th 2016, Chemical Terms, 16.1.11
New features and improvements
-
Improving the following Chemical Terms functions to handle atom index arrays as input parameters: formalCharge(), hCount(), connections(), valence(), radicalCount(), atno(), map().
Bugfixes
-
Improving the matchCount() function to handle molecules with query Hs.
December 14th 2015, Calculator Plugins, 15.12.14
Bugfixes
-
Bugfix for the Tautomerization Plugin: the plugin gave back different canonical tautomers from what were expected for oxim-type molecules.
-
Bugfix for the NMR Plugin: calculating NMR spectra for highlighted molecules did not work.
November 9th, 2015, Calculator Plugins, 15.11.9
Bugfixes
-
Bugfix for the Refractivity Plugin: refractivity failed for a molecule throwing exception. See theforum topic for details.
-
Bugfix for the pKa Plugin: inconsistent calculated values for the same molecule represented in different atom order. See the forum topic for details.
-
Bugfix for the 3D Alignment Plugin: molecule selecting function did not work properly during the alignment process. See the forum topic for details.
November 2nd, 2015, Calculator Plugins, 15.11.2
Bugfixes
-
Bugfixes for the Tautomer Plugin: bugs in the dominant tautomer distribution calculation for molecules containing P. See the forum link for details.
-
Bugfix for the Solubility Predictor: calculated solubility at pH 7.4 was not the same in cxcalc and MarvinSketch
October 26th, 2015, Calculator Plugins, 15.10.26
Bugfixes
October 12th, 2015, Chemical Terms, 15.10.12
Bugfixes
- Bugfix for calculating chemical properties for agents in ReactionContext: evaluating Chemical Terms functions for reaction agents threw Exception
October 5th, 2015, Calculator Plugins, 15.10.5
Bugfixes
-
Bugfix for the pKa calculation: improving calculated values in relation to the log D training. See the forum link for details.
-
Bugfixes for the Tautomer Plugin: bugs in the dominant tautomer distribution calculation in cxcalc. See the forum link for details.
-
Bugfix for the steric hindrance calculation in Metabolizer: slow-down of generation of 3D conformers for molecules with fused rings fixed. See the forum link for details.
September 14th, 2015, Calculator Plugins, 15.9.14
Bugfixes
- Bugfix for the MCS-based Alignment method in the Alignment Plugin. See the forum link for details.
August 24th, 2015, Calculator Plugins, 15.8.24
Bugfixes
-
Bugfix for major microspecies calculation in cxcalc: fix for lost SD properties in the output.
-
Bugfix for the log P /log D plugin: correct error message shown for cation/anion concentration setting
August 10th, 2015, Calculator Plugins, 15.8.10
Bugfixes
-
pKa calculation fixes for amide structures. See the forum link for details.
-
Fix for solving the pKa calculation slow-down and JVM break.
August 3rd, 2015, Calculator Plugins, 15.8.3
New features and improvements
-
Calculating chemical properties for highlighted structures: it is possible to run the calculators for highlighted structures in MarvinSketch.
-
Solubility results/plot is automatically updated when the molecule is modified on the canvas in MarvinSketch.
-
New HLB node in KNIME is now available. See the KNIME release notes for details.
July 20th, 2015, Calculator Plugins, 15.7.20
Bugfixes
- Bugfix for tautomerization: tautomer generation for molecules with explicit Hs wasn't working properly.
July 13th, 2015, Calculator Plugins, 15.7.13
Bugfixes
June 29th, 2015, Calculator Plugins, 15.6.29
Bugfixes
-
Bugfixes for tautomerization: various bugs in the tautomerization of the Standardizer KNIME node; tautomerization of indazole for its pKa calculation was incorrect.
-
Bugfix for geometry optimization with MMFF94 in the Geometry Plugin
June 8th, 2015, Calculator Plugins, 15.6.8
Bugfixes
June 1st, 2015, Calculator Plugins, 15.6.1
Bugfixes
-
Bugfix for the Tautomer Plugin: bad oxo-enol tautomerization for a molecule.
-
Bugfix for the Tautomer Plugin: calculating dominant tautomer distribution in cxcalc didn't behave well for certain molecules.
May 18th, 2015, Calculator Plugins, 15.5.18
New features and improvements
- Releasing the new HLB Predictor in MarvinSketch. See the documentation here.
Bugfixes
- Bugfix for calculating the pKa for carbanions. See the forum topic about the bug here.
April 27th, 2015, Calculator Plugins, 15.4.27
New features and improvements
- Releasing the new HLB Predictor in cxcalc, its API and in Chemical Terms. See the documentation here.
April 13th, 2015, Calculator Plugins, 15.4.13
New features and improvements
-
Improving and modifying the tautomerization options in the Tautomerization Plugin. See the documentation for details.
-
Improving and modifying the log P calculation methods in the log P Plugin. See the documentation for details.
-
Improving and modifying the log D calculation methods in the log D Plugin See the documentation for details.
March 23rd, 2015, Calculator Plugins, 15.3.23
Bugfixes
March 16th, 2015, Calculator Plugins, 15.3.16
Bugfixes
March 9th, 2015, Calculator Plugins, 15.3.9
Bugfixes
February 16th, 2015, Calculator Plugins, 15.2.16
Bugfixes
February 9th, 2015, Calculator Plugins, 15.2.9
Bugfixes
February 2nd, 2015, Calculator Plugins, 15.2.2
Bugfixes
January 19th, 2015, Calculator Plugins, 15.1.19
Bugfixes
January 5th, 2015, Calculator Plugins, 15.1.5
New features and improvements
- Stiochiometry in chemical formulae is shown in subscripts. Subscripts are now correctly displayed in the Elemental Analysis Plugin.
December 15th, 2014, Calculator Plugins, 14.12.15
Bugfixes
December 8th, 2014, Calculator Plugins, 14.12.08
New features and improvements
Bugfixes
November 24th, 2014, Chemical Terms, 14.11.24
Bugfixes
November 17th, 2014: Calculator Plugins, 14.11.17
Bugfixes
November 3rd, 2014: Calculator Plugins, 14.11.03
New features and improvements
Bugfixes
October 20th, 2014: Calculator Plugins, 14.10.20
Bugfixes
October 13th, 2014: Calculator Plugins 14.10.13
Bugfixes
- Setting of the measurement unit of the Solubility Predictor (unit could not be set in MSketch, issue fixed)
September 22nd, 2014: Calculator Plugins 14.9.22
New features and improvements
- Chemical Terms stereoAnalysis() function has been improved. Documentation.
September 15th, 2014: Calculator Plugins 14.9.15
New features and improvements
-
New Solubility Predictor API has been released. API doc.
-
Chemical Terms logS() function has been improved. Documentation.
August 18th, 2014: Calculator Plugins 14.8.18
New features and improvements
-
New calculation in Elemental Analyser Plugin: mass spectrum (Documentation).
-
'Grouped dot disconnected formula' has been added to Elemental Analyser Plugin.
August 11st, 2014: Calculator Plugins 14.8.11
Bugfixes
-
Bugs reactant() and product() functions in Chemical Terms have been fixed.
-
'Setting radius' option in Geometry Plugin has been removed.
Please visit this page for History of changes relating older versions of Calculator Plugins.